zn 2+ electron configuration|Zinc : Pilipinas March 23, 2023. Electron configuration chart of all Elements . Get the latest breaking news and stories in the Philippines and around the world from GMA News Online.
PH0 · Zinc Electron Configuration:7 Easy Step
PH1 · Zinc
PH2 · What is the electron configuration for Zn^2+? Chemistry Q&A
PH3 · What is the electron configuration for Zn^2+? Chemistry Q&A
PH4 · What is the electron configuration for Zn2+?
PH5 · Electron Configuration for Zn and Zn2+ (Zinc and Zinc
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Electron Configuration Chart
PH8 · Complete Electron Configuration for Zinc (Zn, Zn2+ ion)
PH9 · 3.1: Electron Configurations
sites can educate, entertain, or provide users with practical services. sites can also be a means of artistic expression on or a platform to deliver wholly unique and unusual experiences. Our weird websites list reflects some of the most surprising and funny sites you’ll find online—ranging from practice to pointless. 50 .
zn 2+ electron configuration*******Alternatively you can use a chart showing how the orbitals fill ( • Using the Electron Configuration Chart ). Either way, the Zinc electron configuration will be 1s2 2s2 2p6 3s2 3p6 3d10. March 23, 2023. Electron configuration chart of all Elements .
Updated on February 01, 2021. The electron configuration of .
zn 2+ electron configuration Zinc Electronic configuration of Zinc ion Zn 2 +: Zn loses its two electrons and results in the formation of Zn 2 + ion. The electronic configuration of the Zinc ion is 1 s 2 2 s 2 2 p 6 3 .
The electronic configuration of Zn is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 and is represented below, where. In s orbital 2 electrons accommodate. In the p orbital, 6 .
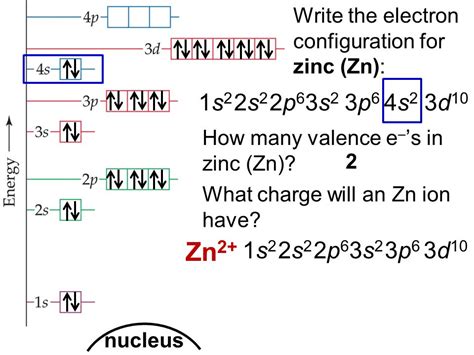
3.1: Electron Configurations. Page ID. Skills to Develop. Derive the predicted ground-state electron configurations of atoms. Identify and explain exceptions to predicted electron configurations for atoms and .

3.1: Electron Configurations. Page ID. Skills to Develop. Derive the predicted ground-state electron configurations of atoms. Identify and explain exceptions to predicted electron configurations for atoms and .Electron configuration 3d 10 4s 2: Electrons per shell: 2, 8, 18, 2: Physical properties; Phase at STP: solid: Melting point: 692.68 K (419.53 °C, 787.15 °F) Boiling point: 1180 K (907 °C, 1665 °F) Density (near r.t.) 7.14 .Electron configuration. The arrangements of electrons above the last (closed shell) noble gas. Melting point. The temperature at which the solid–liquid phase change occurs. .
The electronic configuration for Cl-can, therefore, be designated as 1s 2 2s 2 2p 6 3s 2 3p 6. Again, the electron configuration for the chloride ion is the same as that for Ca 2+ and Argon. Hence, they .
Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. . Represented in the periodic table as Zn, zinc is a transition metal, grouped with cadmium and mercury. With the middling atomic number 30, it has five stable isotopes of . Zn2+ is not a neutral molecule but instead a charged molecule. The +2 charge on the zinc atom indicates that it has lost two electrons. Hence +2 is the charge carried by the zinc atom. Count the valence count for Zn2+ Zinc has electronic configuration of [Ar] 3d¹⁰4s² whereas for zn2+ , electronic configuration corresponds .
The full electron configuration of zinc is 1s2 2s22p6 3s23p63d10 4s2Zinc, also written zinc, is defined as the chemical element that belongs to the periodic table of elements. It is located in group 12, its symbol is Zn and its atomic number is 30. This element is a metal. it is sometimes classified as a transition metal, although basically .
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .Zinc. Full electron configuration of zinc: 1s2 2s2 2p6 3s2 3p6 3d10 4s2. copper ← zinc → gallium.
In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule.
Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3s 2 3p 1, is analogous to its family member boron, [He]2s 2 2p 1. . (Zn, Cd, Hg, as well as Cu, Ag, and Au in Figure 6.29) are not technically transition elements.The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).The electron configuration of a neutral zinc atom is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 1 0 4 s 2. The Z n 2 + ion has lost two electrons, which leaves it with 3 0 protons and 2 8 electrons. The electron configuration of Z n 2 + is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 1 0 .zn 2+ electron configuration The atomic number of Zinc (Zn) is 30. Therefore, the electronic configuration of Zinc can be represented as: 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Here, the first shell contains 2 electrons (1s²), the second shell contains 8 electrons (2s² 2p⁶), the third shell contains 18 electrons (3s² 3p⁶), and the fourth shell contains 2 electrons in the s .The atomic number of zinc is $$30$$, which means that the neutral atom has equal numbers of protons and electrons, so a neutral atom of zinc would have $$30$$ electrons. The electron configuration of a neutral zinc atom is $$1s^{2}2s^{2}2p^{6}3s^{2}3p^{6}3d^{10}4s^{2}$$.Write the electron configurations of these cations. Solution. First, write the electron configuration for the neutral atoms: Zn: [Ar]3 d10 4 s2. Cr: [Ar]3 d5 4 s1. Next, remove electrons from the highest energy orbital. For the transition metals, electrons are removed from the s orbital first and then from the d orbital.
Zinc represents the symbol Zn in the periodic table. Let us discuss about electronic configuration of zinc in this article. The electronic configuration of zinc is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10.Electrons are distributed among the shells K, L, M, and N. Zn is the 12 th group element and one of the transition metals in the periodic table.. We .Zinc Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structure.The chemical symbol for Zinc is Zn. Electron Configuration and Oxidation States of Zinc. Electron configuration of Zinc is [Ar] 3d10 4s2. Possible oxidation states are +2. Electron Configuration. The periodic .The zinc atom exhibits a Zn 2+ ion. The zinc atom donates two electrons in the 4s orbital to form a zinc ion(Zn 2+). Zn – 2e – → Zn 2+ Here, the electron configuration of zinc ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10. This electron configuration shows that zinc ion(Zn 2+) has three shells and the last shell has eighteen electrons. For this .
Mencircle is the leading pinoy gay online community in the Philippines featuring pinoy gay stories and helpful gay related articles with a live video chat room exclusive to Filipino gay men, bisexuals and transgenders worldwide. Mencircle aims to provide a venue for conversation and exchange ofThe McGranahan Barn is a rustic replica of an 1800s barn located just outside of Oklahoma City. This wedding venue has a maximum capacity of 350 guests. You can select from two indoor and two outdoor spaces. The Downstairs and the Loft are elegant indoor spaces with stunning chandeliers and a bar made from the original .
zn 2+ electron configuration|Zinc